Abstract
Introduction:
Primary refractory or relapsed (R/R) acute myeloid leukemia (AML) is associated with poor prognosis. Therapeutic resistance is the principal cause of treatment failure. Despite recent advances in therapeutics, there are still unmet needs in R/R AML. With VIALE-A study (DiNardo et al., 2020), azacitidine-venetoclax (AZA-VEN) has been used more frequently in current practice for R/R disease without strong evidence to support its use after first induction. The purpose of this study was to report patient selection and outcomes in adults who received AZA-VEN compared to other therapies (NO-AZA-VEN cohort) during the same time period.
Methods:
Between January 1st 2019 and January 1st 2021, we retrospectively analyzed the charts of 84 consecutive patients who had R/R AML, of whom 68 (81%) received at least one line of chemotherapy for R/R disease. Nine patients were excluded because they previously received an hypomethylating agent. Of the 59 patients analyzed, 20 (34%) received AZA-VEN and 39 (66%) received other therapies.
Results:
The baseline characteristics are described in Table 1. Median age of patients was 60 years old and 51% were males. Second line of treatment (first line for R/R setting) for the NO-AZA-VEN cohort was either NOVE (51%), FLAG-IDA (10%), IDAC (13%), Sorafenib (15%), HDAC-12 (5%), CPX-351 (3%) or Gilteritinib (3%). In the AZA-VEN cohort, 6 (30%) patients received it in second line, 10 (50%) patients in third line and 4 (20%) in fourth line. There was significantly more European LeukemiaNet (ELN) 2017 adverse risk patients in the AZA-VEN cohort compared to the NO-AZA-VEN cohort (55% vs 5%, p<0.001). However, there was no FLT3-ITD mutations in the AZA-VEN cohort compared to 11 patients (28%) in the other cohort, p=0.01. For those who achieved a complete response (CR) after first induction (n=40), the median time to relapse was 21 months in the AZA-VEN cohort and 17 months in the NO-AZA-VEN cohort, p=0.229. There were 9 (45%) patients in the AZA-VEN cohort who received an allogenic hematopoietic stem cell transplant in first remission compared to 25 (64%) patients in the NO-AZA-VEN cohort, p=0.177.
Median follow up was comparable in both groups with 8 months in the AZA-VEN cohort compared to 7 months in the NO-AZA-VEN cohort, p=0.521. From the date of relapse or the date of refractory disease after first line therapy, the AZA-VEN cohort had a median overall survival (OS) of 8 months compared to 9 months in the NO-AZA-VEN cohort, p=0.854.
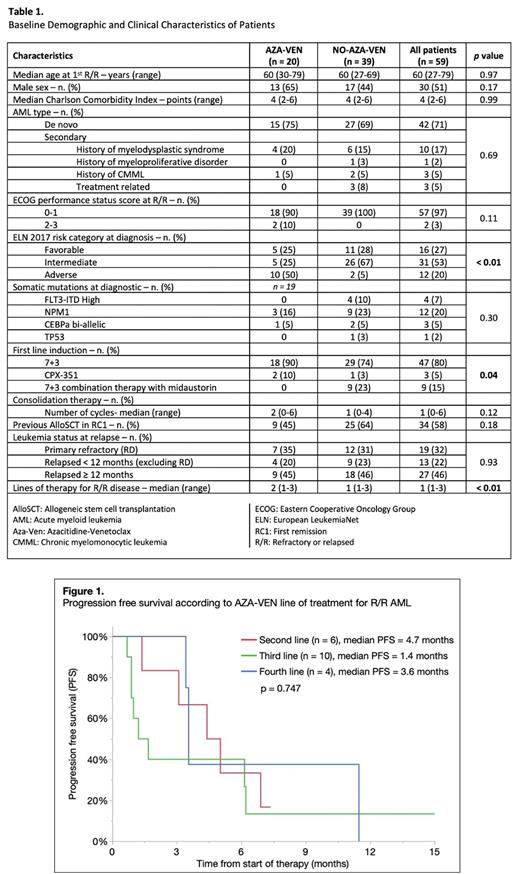
Receiving AZA-VEN in second, third and fourth line was associated with an overall response rate (ORR), composed of CR and CR with incomplete hematologic recovery (CRi), of 67% (n=6), 50% (n=10) and 50% (n=4), respectively and a median progression free survival (PFS) of 4.7, 1.4 and 3.6 months, p=0.747 (Figure 1).
Most frequent causes of death for both AZA-VEN and NO-AZA-VEN cohorts were leukemia progression (64% vs 54%, p=0.739) and infection (21% vs 42%, p=0.299). There was no statistical difference in complications of treatment such as number of hospitalization days (median 38 days vs 32 days, p=0.637), febrile neutropenia (85% vs 90%, p=0.680), intensive care unit stay (5% vs 8%, p=1.00), septic shock (10% vs 5%, p=0.560) and total parenteral nutrition (0% vs 13%, p=0.156).
Although the cohort is small for subgroup analyses, age, Eastern Cooperative Oncology Group (ECOG) performance status score, line of treatment, ELN 2017 risk categories, AML subtype and previous allogenic hematopoietic stem cell transplant did not impact ORR, PFS and OS in the AZA-VEN cohort.
Conclusion:
Use of AZA-VEN was associated with good ORR despite being used in later line of treatment, but with a short mean of PFS. Even though AZA-VEN was used with more adverse risk R/R AML, the overall survival was comparable to the NO-AZA-VEN cohort. Further randomized studies are needed to advise on the usage of AZA-VEN in the R/R setting.
Disclosures
Munger:Bristol Myers Squibb: Consultancy; AbbVie: Consultancy. Marcoux:Bayer: Consultancy; Ipsen: Consultancy; Novartis: Consultancy; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy; Pfizer: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau. Lemieux:AbbVie: Consultancy, Speakers Bureau.
OffLabel Disclosure:
Retrospective study of azacitidine + venetoclax in refractory or relapsed (R/R) acute myeloid leukemia. This drug combination is only studied in first line of treatment. There is no "official" indication for its use in R/R disease.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal